In the field of molecular diagnostics, nucleic acid testing with an ultra-low limit of detection is of critical significance. It enables the transformation of pathogen detection and disease management from passive response to proactive prediction and personalized medicine.
On November 19, 2025, a long review titled Ultrasensitive Nucleic Acid Testing: From Foundational Research to Clinical Translation was jointly published in the top journal ACS Nano by the team led by Researcher Liu Peng from the School of Biomedical Engineering, Tsinghua University, and the team of Professor Chwee Teck Lim from the National University of Singapore. The review systematically sorts out the technical paths, cutting-edge advances, clinical application challenges and future directions in this field.

Click here for more paper details (Link:https://doi.org/10.1021/acsnano.5c15324 )
The review highlights the major breakthroughs in the 21st century driven by microfluidics, nanotechnology, and artificial intelligence, structured around three core paradigms: first, how amplification-dependent platforms (e.g., digital PCR, isothermal amplification) have reshaped the precision and speed of quantitative detection; second, how amplification-free technologies (e.g., CRISPR-based diagnostics, nanopore sequencing) enable direct detection of native molecules; and third, how nanotechnology-driven biosensors convert biorecognition events into highly sensitive physical signals.Meanwhile, the review addresses key bottlenecks in ultrasensitive nucleic acid testing, including the standardization of sample pretreatment and the integration of "sample-in, result-out" systems, while providing insights to support the future development of decentralized and intelligent diagnostics. Finally, it emphasizes that the clinical translation of future technologies must align with the WHO’s "ASSURED" criteria, ensuring the realization of affordable, sensitive, specific, user-friendly, rapid, robust, and grassroots-deployable diagnostic capabilities.

Figure 2:Graphical Abstract
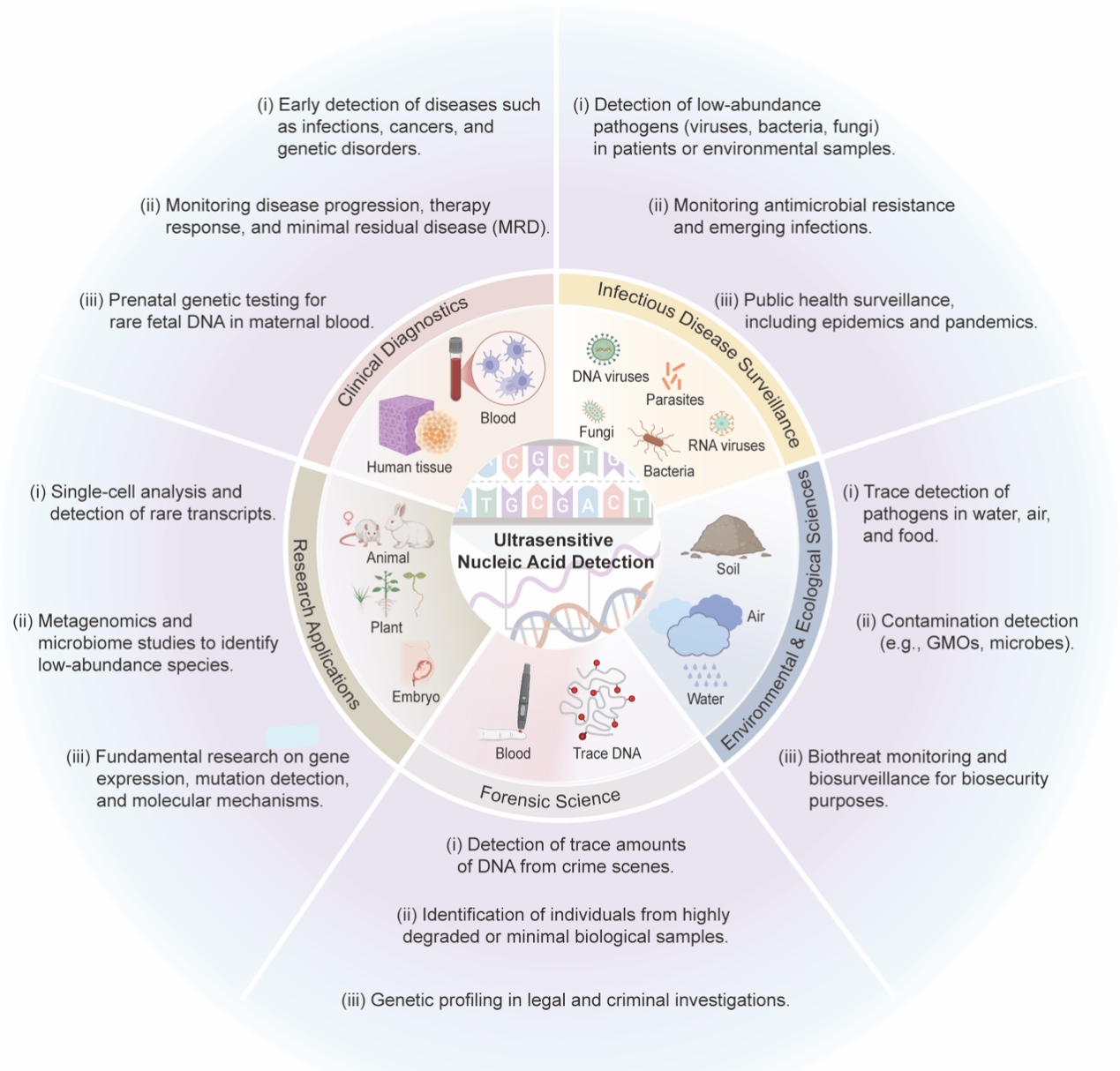
Figure 3:Key Application Scenarios of Ultrasensitive Nucleic Acid Testing Technologies
Amplification-Dependent Platforms: As the most mature technology applied in clinical practice, this type of testing relies on enzymatic reactions to exponentially replicate trace target molecules, thereby "amplifying" the signal itself. The review mainly discusses digital PCR, enzymatic isothermal amplification, enzyme-free cascade amplification and other technologies.
Amplification-Free Paradigms: Unlike amplification-based technologies, this paradigm directly detects native nucleic acid molecules without exponential replication of targets. Representative technologies include CRISPR-Cas systems and nanopore sequencing.
Nanotechnology-based Biosensors: With their high specific surface area, quantum confinement effects and unique optoelectronic properties, nanomaterials serve as ideal signal transducers linking biorecognition events to macroscopically readable signals, instantly converting biological recognition into measurable physical signals. Specific technologies include Surface-Enhanced Raman Scattering (SERS), Fluorescence Resonance Energy Transfer (FRET), and Field-Effect Transistors (FETs).
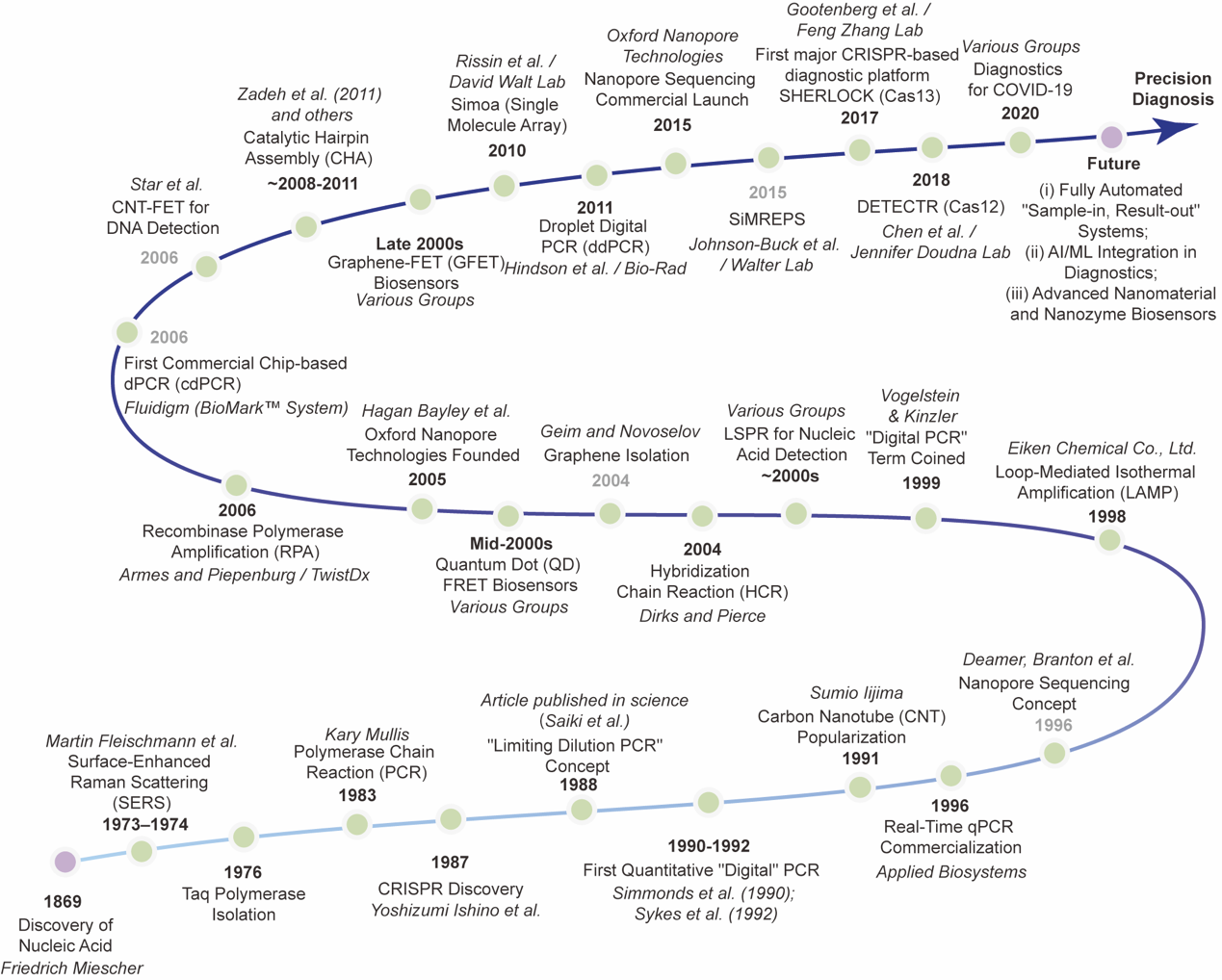
Figure 4:Over 150 Years of Technological Evolution in Nucleic Acid Testing
Since Friedrich Miescher discovered nucleic acids in 1869, nucleic acid testing technologies have undergone a leap from qualitative to quantitative detection, and further to digital single-molecule detection over the past 150-odd years. The continuous emergence of technologies such as digital PCR, isothermal amplification, CRISPR-based diagnostics and nanopore sequencing has endowed us with unprecedented ultra-sensitive detection capabilities. As these technologies mature, we are expected to break the limitations of traditional laboratories, usher in a new era of intelligent, automated and decentralized healthcare. This will facilitate the development of personalized medicine, promote public health, and build a truly healthy, intelligent and inclusive future medical ecosystem.
Li Bao, Gu Xuanyu and Zeng Wu from the School of Biomedical Engineering, Tsinghua University, are the co-first authors of this review. Researcher Liu Peng from the School of Biomedical Engineering, Tsinghua University, and Professor Chwee Teck Lim from the National University of Singapore, are the co-corresponding authors.


