Liver cirrhosis is a severe chronic liver disease, with portal hypertension as one of its key features. Vascular system injury in the liver caused by portal hypertension triggers a series of life-threatening complications such as ascites, rupture and bleeding of esophagogastric varices, resulting in approximately 1.2 million deaths worldwide each year. Currently, there are no FDA-approved curative drugs except liver transplantation. For a long time, due to the lack of in vitro models that can simulate the complex mechanical microenvironment around blood vessels in cirrhotic conditions, the pathological mechanism by which abnormal hydrostatic pressure leads to vascular injury has remained a bottleneck in the field.
On November 14, 2025, the research group led by Professor Du Yanan from the School of Biomedical Engineering, Tsinghua University, published a research paper entitled "Hepatic hypertension on-a-chip identifies GPR116 as a hydrostatic pressure mechanosensor to regulate vascular injury in cirrhosis" in the journal Science Advances. The study successfully developed two-dimensional (2D) static and three-dimensional (3D) dynamic "Hepatic Hypertension-on-a-Chip (HH-Chip)" models, which for the first time simulated the hemodynamic and matrix mechanical properties of liver cirrhosis in vitro, and revealed the key mechanism by which mechanical forces drive vascular injury during cirrhosis progression. Using this innovative platform, GPR116 was identified as a mechanosensor for hydrostatic pressure in liver sinusoidal endothelial cells (LSECs), and its downstream signal transduction pathway was clarified. Furthermore, the therapeutic potential of targeting GPR116 in delaying cirrhosis progression was verified through cell therapy and gene therapy. This discovery not only provides a new perspective for understanding the pathology of liver cirrhosis but also paves the way for the development of novel therapies targeting anti-vascular degeneration rather than traditional anti-angiogenesis, which is expected to improve the treatment dilemma of patients with advanced liver cirrhosis.
HH-Chip Recreates the Complex Mechanical Microenvironment of Liver Cirrhosis
The HH-Chip system designed by the research team includes two configurations: 2D static and 3D dynamic (Figure 1). The 2D static chip cultivates LSECs on hydrogels of different stiffness in a PDMS pressure chamber and applies hydrostatic pressure within a physiological to pathological range, enabling precise observation of cell behaviors such as morphology, migration, and adhesion. The study found that when LSECs are exposed to high pressure on a high-stiffness matrix, they exhibit vascular degeneration phenotypes similar to cirrhosis, such as a sharp decrease in cell number and contractile aggregation.
The more sophisticated 3D dynamic chip simulates changes in matrix viscoelasticity during cirrhosis by regulating the crosslinking degree of type I collagen, and integrates a fluid resistor to precisely control intravascular pressure while maintaining physiological shear stress. This model successfully reproduced the different vascular injury responses of LSECs to high pressure in soft/hard blood vessels, which is highly consistent with the findings of the 2D chip and in vivo pathological features.
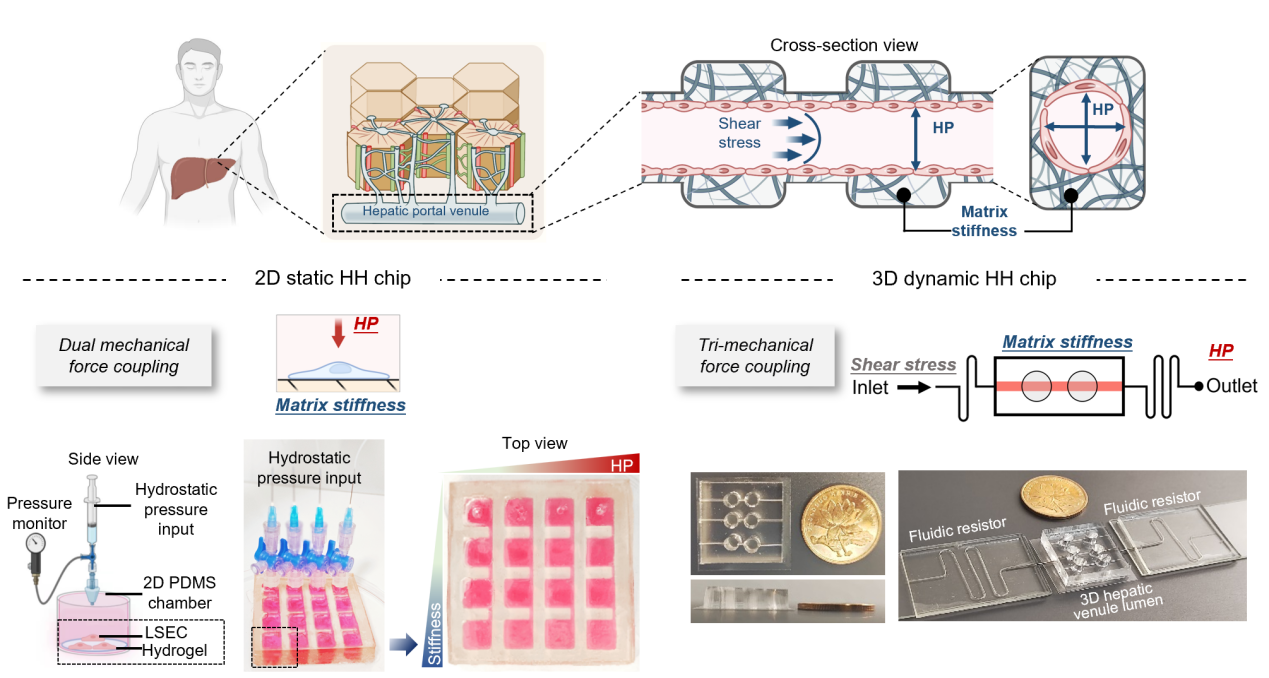
Figure 1: Design of the Hepatic Hypertension-on-a-Chip (HH-Chip)
HH-Chip Screens and Identifies GPR116 as the "Sentinel" for High Pressure Sensing
Using the HH-Chip platform, a three-stage screening was conducted: 1) Screening 8 membrane protein genes with specific changes in expression trends under high-pressure stimulation in the 2D chip; 2) Locking genes specifically expressed in liver vascular endothelium by combining with human cirrhotic single-cell sequencing databases; 3) Verifying in human and mouse cirrhotic samples. Finally, GPR116, an adhesion GPCR protein with unknown function previously, was identified as the key pressure-sensing membrane protein.
Subsequent functional experiments confirmed that after knocking down GPR116 in LSECs, the cells showed significantly reduced apoptosis and senescence under the combined effect of high-stiffness matrix and high pressure, maintained the cell sieve pore structure, and protected the integrity of the 3D vascular barrier (Figure 2).

Figure 2: GPR116 acts as a hydrostatic pressure mechanosensor to regulate vascular injury in liver cirrhosis
Further in-depth analysis of GPR116's force sensing and signal transduction mechanism revealed that GPR116 specifically responds to pressure changes, rather than shear force, tensile force, or matrix stiffness changes. Overexpression of GPR116 in LSECs or HEK 293T cells can activate downstream signals under pressure; conversely, knocking down GPR116 can effectively protect cells from high-pressure-induced injury, a property not possessed by other known mechanosensors such as PIEZO1, PIEZO2, and TRPV4. When sensing pressure, GPR116 undergoes autohydrolysis, activates the downstream Gs signaling pathway, thereby triggering cytoskeletal disorders, inhibiting the PI3K/Akt signaling pathway and the phosphorylation of CREB protein, and ultimately leading to cell apoptosis.

Figure 3: Schematic diagram of the mechanism of GPR116 for mechanosensing and mechanotransduction
New Therapeutic Strategy: From Anti-Angiogenesis to Anti-Vascular Degeneration
Based on the mechanism research, the team explored two therapeutic strategies targeting GPR116. On one hand, transplanting shGPR116-LSECs resistant to high-pressure injury into the fibrotic livers of mice via splenic injection found that these cells could reduce collagen deposition and promote vascularization to a certain extent, demonstrating the prospect of this engineered liver sinusoidal endothelial cell therapy. On the other hand, delivering shGpr116 via AAV2/9 viral vector in the early stage of fibrosis successfully reduced GPR116 expression in vivo and delayed the progression of liver fibrosis to advanced stages, confirming the feasibility of gene therapy.
In current cirrhosis treatment, non-selective β-blockers can reduce portal pressure but have side effects such as causing hypotension and increasing the risk of death. This study proposes a paradigm shift: the intervention strategy targeting GPR116 aims to "maintain" vascular health and prevent its degeneration, rather than simply "blocking" angiogenesis. As an orphan receptor, GPR116 is not involved in regulating vascular contraction and relaxation, so targeting it may provide a safer hypotensive approach than existing drugs.
This study not only provides the HH-Chip platform for cirrhosis research, overcoming the limitations of traditional models in simulating complex mechanical microenvironments, but more importantly, reveals for the first time the novel role of GPR116 as a liver vascular-specific pressure mechanosensor and systematically clarifies its core mechanism in cirrhotic vascular injury. The proposed new cell and gene therapy strategies targeting GPR116 open up new directions for the prevention and treatment of liver cirrhosis. Notably, as a universal platform, HH-Chip is expected to be widely used in pathological research, target discovery, and drug screening for other high-pressure-related diseases (such as pulmonary hypertension, atherosclerosis, hypertensive retinopathy, and nephropathy), showing broad translational potential.
Professor Du Yanan from the School of Biomedical Engineering, Tsinghua University, who is also aPI at the Tsinghua-Peking University joint Center for Life Sciences (CLS), is the corresponding author of the article. Postdoctoral Long Yi, doctoral students Liang Kaini and Niu Yudi from the School of Biomedical Engineering, Tsinghua University, are the co-first authors. Doctoral student Wang Rui from Peking Union Medical College, Dr. Liu Ruiquan from Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Dr. Zhang Yan from the School of Medicine, Tsinghua University, doctoral students Ao Yanxiao, Jin Yuhong, Wu Zhaozhao, Wu Bingjie, Liu Zhiqiang, and doctoral student Zhang Xin from the School of Pharmacy made important contributions to this research. Professor Liu Bin from the First Affiliated Hospital of Kunming Medical University, Professor Qi Xiaolong from Zhongda Hospital Affiliated to Southeast University, and Professor Liu Xiangyu from the School of Pharmacy, Tsinghua University, provided important guidance for this research. This study was supported by the National Natural Science Foundation of China (NSFC) Distinguished Young Scholar Program, Key Program, and Young Scientist Program Class C (82125018, 32430058, 32401086), the Beijing Natural Science Foundation (Z230016), and the China Postdoctoral Science Foundation General Program (2022M721862).
Article link: https://www.science.org/doi/10.1126/sciadv.adu7596


