On November 21, 2025, a research team led by Prof. Du Yanan from the School of Biomedical Engineering (BME) and Tsinghua-Peking University Center for Life Sciences (CLS) at Tsinghua University published an online research paper titled "Artificial Cells with Liquid-liquid Phase Separation-Regulated Cell-free Protein Synthesis" in Proceedings of the National Academy of Sciences of the United States of America (PNAS) (click here for more paper details. The study developed a physical switch based on liquid-liquid phase separation (LLPS) to construct artificial cells with environment-responsive gating. This work not only presents a novel strategy for regulating cell-free protein expression but also provides preliminary validation for the translation of artificial cells from in vitro experiments to in vivo applications.
1. Research Background: The Gap from In Vitro to In Vivo
Bottom-up synthetic biology aims to simulate and even surpass natural cell functions by constructing artificial cells. Among various technologies, cell-free protein synthesis (CFPS) systems have emerged as a core engine driving artificial cell tasks due to their openness and programmability. However, deploying these in vitro high-performance artificial cells into complex in vivo environments—enabling them to reliably sense, respond, and execute tasks—remains a major challenge in the field. Specifically, the reliability of triggering, in vivo stability, signal readout, and synthetic consistency collectively pose significant hurdles.
2. Equipping Artificial Cells with a Physical Switch: Reversible Gating via Phase Separation
Inspired by the functional abnormalities caused by the aggregation of certain natural cell proteins (e.g., Tau protein) under pathological conditions, the team designed and synthesized a functional biomimetic polymer—carboxyethyl dextran (CE-Dex). This polymer carries pH-responsive charges and can reversibly induce LLPS of the entire CFPS system through multivalent electrostatic interactions, forming micrometer-scale aggregates. In the aggregated state, protein synthesis is physically turned off; when the environmental pH increases to alkaline levels (e.g., specific pathological microenvironments), the condensates rapidly dissociate within seconds, reactivating protein synthesis (Figure 1).
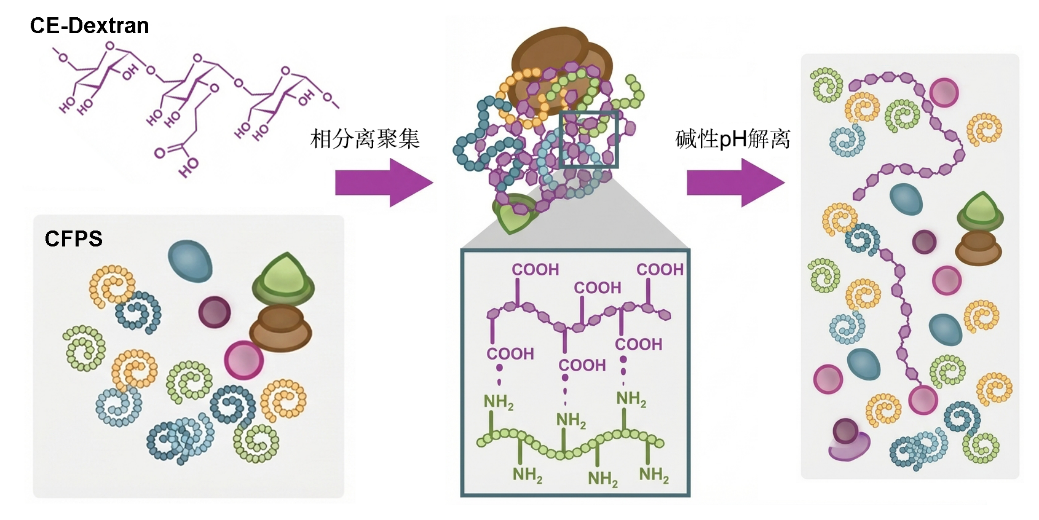
Figure 1 CFPS forms aggregates via intermolecular forces induced by CE-Dex, shutting down protein synthesis; re-dissociation into solution under alkaline pH restarts protein synthesis.
This biophysical, non-genetic switch provides a novel physicochemical means for precise and intelligent regulation of artificial cell functions, featuring rapid response, high reversibility, and a large on/off ratio.
3. Microfluidic Fabrication and Functionalization of Highly Uniform Artificial Cells
To encapsulate the above aggregation-dissociation CFPS system, an advanced microfluidic engineering platform was developed for high-throughput, stable preparation of giant unilamellar vesicles (GUVs) as the carrier framework of artificial cells. Through precise regulation of chip geometry and surface hydrophobicity-hydrophilicity, the platform achieves efficient encapsulation of high-osmolarity CFPS systems under mild, low-surfactant conditions, producing GUVs with highly uniform sizes (Figure 2).

Figure 2 Microfluidic-based micrometer vesicle preparation system for encapsulating CFPS systems, combined with α-hemolysin to enable internal-external communication of artificial cells.
Furthermore, to facilitate information and material exchange between the encapsulated CFPS system and the external environment (i.e., pH sensing and energy substrate acquisition), α-hemolysin (α-HL) nanopores were embedded in the GUV membrane. These pores allow free passage of small molecules such as ions and amino acids while retaining core components required for internal protein expression, enabling controlled internal-external communication.
4.Integration of Phase Separation Systems with Phospholipid Membranes: Controllable Switching in Artificial Cells

Figure 3 Artificial cells achieve pH-responsive LLPS aggregation and dissociation of the CFPS system.
After successfully constructing the LLPS-regulated CFPS system and GUV carrier framework, a critical question needed to be addressed before in vivo application: Would this sophisticated physical switch function normally when encapsulated in a micrometer-scale confined space? To answer this, the team directly observed the internal state of artificial cells using high-resolution fluorescence microscopy. Fluorescently labeled CFPS systems clearly confirmed the aggregation and dissociation processes (Figure 3).
In the presence of CE-Dex inducer molecules, bright, dense fluorescent aggregates formed inside artificial cells—indicating successful activation of the LLPS physical switch, which locked the protein synthesis system in a dormant state. Subsequently, when artificial cells were transferred to alkaline pH solutions mimicking pathological microenvironments, external pH signals were rapidly sensed via pre-installed nanopores on the vesicle membrane. Microscopic observations showed that internal fluorescent aggregates completely dissolved within a very short time, restoring uniform fluorescence distribution—marking successful activation of the physical switch and transition of the system to an active state.
This key in vitro validation confirmed that the LLPS physical switch remains efficient and reversible in micrometer-scale confined spaces, demonstrating that the constructed artificial cells are not merely a simple combination of switches and carriers but functionally integrated intelligent systems.
5. First Demonstration of Artificial Cells for Precise In Vivo Pathological Microenvironment Reporting
Finally, the team applied these intelligent artificial cells to a real mouse disease model. A bile duct ligation (BDL) mouse model was established, and in vivo studies confirmed a stable local alkaline microenvironment (pH ≈ 8.3) in the intrahepatic bile duct region—providing a natural target for precise triggering of artificial cells. After in situ injection of artificial cells carrying a luciferase reporter gene into the liver lesion area of BDL mice, in vivo bioluminescence imaging results clearly showed:
In BDL mice, artificial cells successfully sensed local alkaline signals, leading to dissociation of internal aggregates, activation of CFPS for luciferase synthesis, and ultimately production of strong, highly localized bioluminescent signals (Figure 4).
In healthy control mice, lacking alkaline environmental triggers, artificial cells remained in a dormant state with no detectable luminescence.

Figure 4 Controllable in vivo expression of artificial cells for disease state reporting.
In summary, these artificial cells can serve as in vivo microenvironment detection and reporting tools, recognizing disease-specific biochemical signals in complex living environments and converting them into readily detectable optical signals. Preliminary biosafety assessments also indicated good in vivo compatibility of the system.
6. Research Significance and Prospects
This study provides new insights for the application of CFPS-based artificial cells in precision medicine. Firstly, it offers a novel non-genetic strategy for regulating protein functions via physical phase separation, featuring rapid response and high reversibility—with potential applications in scenarios requiring dynamic, non-invasive control of complex biological reactions. More importantly, it is the first to validate the feasibility of artificial cells as in vivo diagnostic reporting tools in animal models. Future "artificial cell scouts" could be programmed to sense specific enzymatic signals in tumor microenvironments, inflammatory regions, etc., enabling early and precise localization diagnosis of major diseases. Furthermore, replacing reporter proteins with therapeutic proteins could transform such artificial cells into miniature intelligent drug factories that "treat upon detection," opening new avenues for integrated diagnosis and treatment.
Author Contributions and Funding Support
Prof. Du Yanan from the School of Biomedical Engineering and Tsinghua-Peking University CLS is the corresponding author of the paper. Doctoral students Fan Dongdong and Liang Kaini from Tsinghua’s School of Biomedical Engineering are co-first authors. Dr. Wu Bingjie, Master's graduate Chen Haoke, Postdoctoral Fellow Sun Lei, Dr. Zhang Yan from the School of Basic Medical Sciences, and Bachelor's graduate Sun Chengyu from the School of Biomedical Engineering made important contributions to the research. Prof. Han Fuguo from the School of Pharmaceutical Sciences provided support for related experiments. This work was supported by the National Natural Science Foundation of China (NSFC) Distinguished Young Scholar Program and Key Program (Grants: 82125018, 32430058).


